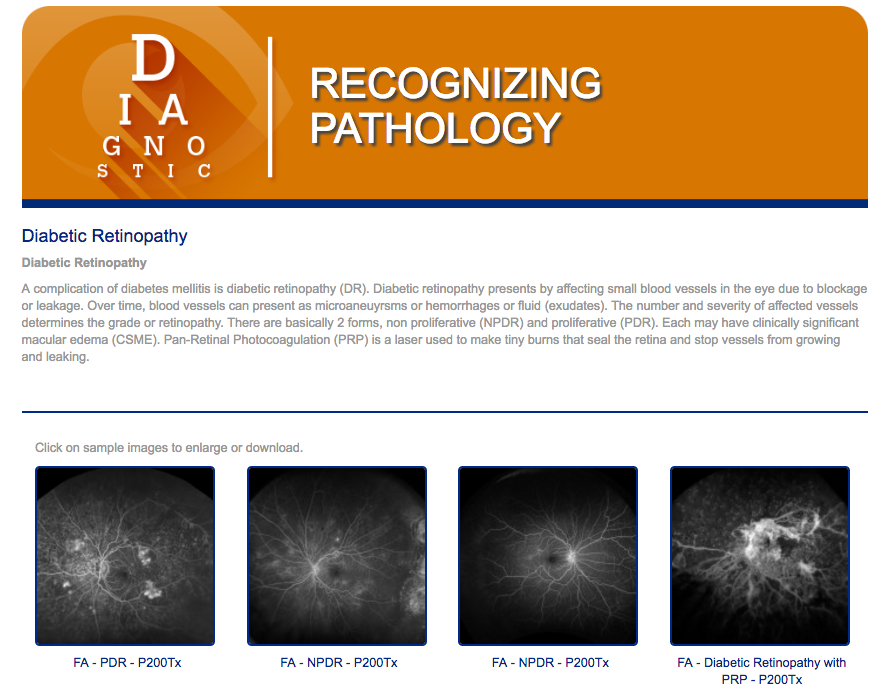
To see images of diabetic retinopathy, visit www.recognizingpathology.optos.com/diabetic-retinopathy/.
Bringing Diagnosis into the 21st Century
Perhaps the biggest change in DR diagnosis and management is the growing use of ultra-widefield imaging.
UWF imaging has been proven in studies to be clinically equivalent to conventional ETDRS seven field color imaging (7SF) in the grading of DR for central pole disease. More recent studies have shown that UWF may be superior to ETDRS in that it captures a much wider (200 degrees), view of the retina. This provides diagnostic information about the peripheral retina that is impossible to visualize using conventional imaging. Starting with color (red and green) optomap® imaging, Optos has systematically extended its UWF-based technology into a multi-modal platform that supports fundus autofluorescence(optomap af), fluorescein angiography(optomap fa) and indocyanine green angiography (optomap icg).
Numerous studies have affirmed how UWF imaging can improve DR and DME management:
— Studies correlating UWF imaging with conventional 7SF imaging have confirmed UWF imaging’s effectiveness in the assessment of DR1-3. Color optomap and optomap fa images show more pathology than 7SF imaging, including peripheral neovascularization, vascular nonperfusion, and microaneurysms. The result is a more complete assessment of DR severity and progression4-6.
— Long term studies have found that peripheral lesions uncovered by UWF imaging were related to a greater risk of DR progression over a four-year period. These findings were not affected by diabetic control or baseline severity of the disease7.
— Other work, using optomap fa, has correlated the presence and extent of peripheral retinal ischemia and DME8. Patients undergoing treatment for DME with higher rates of late peripheral vascular leakage, neovascularization, and nonperfusion were also found to have higher risk of recurrent, post-vitrectomy diabetic vitreous hemorrhage9.
Over the past decade UWF imaging has given both researchers and practitioners a multi-modal diagnostic foundation on which to better (1) measure the extent of DR, (2) assess the risk of progression to more serious forms of DR and, (3) understand the potential for DME post-treatment complications.
Making DR and DME Treatment More Practical and Individualized
A recent roundtable and case study review10 summarized results from a series of recent clinical studies and offered expert guidance on how practitioners can apply these findings. One theme of the roundtable was ways ophthalmologists can design more practical and effective DR and DME treatment plans. The roundtable included discussions of:
— Glycemic Control: Tight glycemic control – in which diabetic patients are encouraged to achieve HbA1c targets in the 6.0% range – may not be appropriate in older patients with a long history of type 2 diabetes and one or more risk factors of cardiovascular disease (CVD). Studies suggest that intensive glycemic control applied to these patients did nothing to improve CVD outcomes.
Related to this, a major study covering almost 3,000 patients over four years found that intensive glycemic control (HbA1c under 6%), could reduce the risk of DR progression, but only in patients with mild retinopathy at the baseline. Patients with no, or moderate to severe retinopathy, saw no benefits.
The roundtable concluded that for patients that can’t benefit from tight glycemic control, their overall risk of DR progression can still be lowered by reducing their HbA1c levels to the 8% to 9% range.
— DME Treatment: Recent studies found that anti-VEGF therapy can deliver positive results for DME patients (improved visual acuity, reduced central retina thickness), at both high and low levels of glycemic control. The roundtable observed that “…it is worth initiating anti-VEGF therapy for patients with DME regardless of their levels of glycemic control.”
The use of laser photocoagulation and sustained release steroid implants was also reviewed. Discussion centered on the indications for use and, for laser therapy, recent research suggesting that prompt initiation of laser therapy after anti-VEGF treatment may have detrimental effects.
— Case Studies: One patient’s presentation included color optomap and optomap fa imaging that revealed diffuse microaneurysms and possible macular ischemia. Diagnosed with severe non-proliferative DR and severe, clinically significant DME, the patient was administered anti-VEGF treatments. While visual acuity improved after multiple injections, edema persisted and the patient received follow-on laser treatment. The patient’s response to follow-on treatments was mixed, illustrating the complexity and uncertainty associated with the treatment of DR and DME.
Overall, the roundtable emphasized that while anti-VEGF therapies have become the first-line therapy for DME, it’s important that practitioners:
— Work with their patients to achieve appropriate levels of diabetic control as a proven means of improving overall DR and DME treatment outcomes.
— Develop individualized treatment plans based on factors that include age, disease duration, CVD risk, life expectancy, and a detailed characterization of the extent of their DR and/or DME.
Improving Patient Outcomes
How are leading practitioners responding to these developments in the treatment and diagnosis of DR and DME?
First, they’re using UWF imaging to give them complete and otherwise unobtainable diagnostic imagery. In particular, they’re analyzing peripheral retina pathology in order to fully assess the extent of disease and risk of progression. Second, they’re developing individual patient management plans that address indicated risks, encourage practical glycemic control, and apply the most effective treatments. Taken together they define an upward path towards continued improvements in patient outcomes.
For More Information:
Tornambe, Paul E., Ultra-Widefield Imaging: Advancing the Understanding and Management of Diabetic Retinopathy, Retina Today, April 2015
Expert Insights in Today’s Multidisciplinary Management of Diabetic Retinopathy and Diabetic Macular Edema; Proceedings from an Expert Roundtable Discussion, A CME Monograph by The University of Louisville Office of Continuing Medical Education and MedEdicus LLC, October 2015
Sources:
6. Silva PS, Cavallerano JD, Sun JK, et al. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120(12):2587-2595.
10. Expert Insights in Today’s Multidisciplinary Management of Diabetic Retinopathy and Diabetic Macular Edema; Proceedings from an Expert Roundtable Discussion, A CME Monograph by The University of Louisville Office of Continuing Medical Education and MedEdicus LLC, October 2015