A recent report describes a unique, high-value application of ultra-widefield (UWF™) retinal imaging in the treatment of Diabetic Macular Edema, or DME.
ILUVIEN® Intravitreal Implant, Charles Mayron MD, FACS Capital Retina Associates PLLC
There are over 400 million people1 worldwide afflicted with diabetes and the long-term incidence of DME in this population is estimated at twenty to forty percent2. UWF imaging is already impacting the diagnosis and treatment of DME as well as Diabetic Retinopathy (DR), which frequently precedes or is associated with DME. Ultra-widefield imaging is unique in that it visualizes a much larger area of the retina (200°, or over 80%) as compared to conventional techniques that may image 45° or less of the retina. By imaging the peripheral retina practitioners and researchers have shown they can more accurately measure the extent of diabetic retinopathy3 as well as better predict the risk of future progression4. UWF is also helping public health initiatives by enabling accurate and cost-effective screening for DR5.
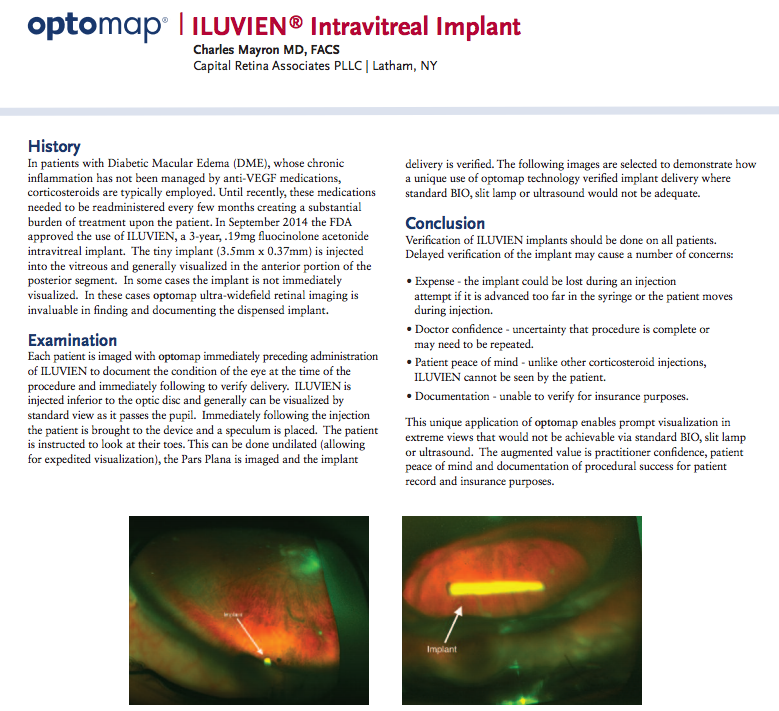
This novel application of UWF imaging supports a relatively new therapy for DME. While the standards for DME treatment are undergoing intense study6, corticosteroids are often prescribed if anti-VEGF medications fail to control the chronic inflammation associated with DME. These corticosteroids are administered by direct injection into the eye, a process that must be repeated every three or four months. In September of 2014 the FDA approved a new method for administering corticosteroids – the Iluvien intravitreal implant. The tiny implant – 3.5 mm long and 0.37 mm in diameter – also requires injection into the eye, but lasts for three years7. This eliminates multiple, uncomfortable patient procedures while providing a continuous application of medication.
The new injectable implant brings some practical issues. First, after injection the location of the implant needs to be verified to assure that procedure was successful. Second, the procedure needs to be documented for insurance purposes.
UWF imaging is being used to address both of these concerns. In a case note, Dr. Charles Mayron reports a technique using UWF color imaging before and after intravitreal implant injection to visualize the implant’s location. What’s notable about the technique is that rather than the usual scanning protocol, patients are instructed to look down and/or move their eye in order to bring the implant into view. The result is a clear snapshot of the implant, without resort to dilation and other, less effective visualization methods like slit lamp, standard BIO, or ultrasound.
This procedure is one more example of how UWF retinal imaging is playing an increasingly important role in the diagnosis and treatment of both diabetic macular edema and diabetic retinopathy.
Sources:
- https://www.diabetesatlas.org/
- https://eyewiki.aao.org/Diabetic_Macular_Edema
- https://www.ncbi.nlm.nih.gov/pubmed/22080911
- https://www.ncbi.nlm.nih.gov/pubmed/25704318
- https://care.diabetesjournals.org/content/early/2013/08/06/dc13-1292.abstract?sid=e2a00219-8738-487d-b45b-0558c446d152
- https://www.omicsonline.org/management-of-diabetic-macular-edema-in-current-clinical-practice-a-review-2155-6156.S3-004.php?aid=479
- https://www.visionaware.org/blog/visionaware-blog/the-fda-approves-injectable-implant-iluvien-for-treatment-of-diabetic-macular-edema-1546/12