More than 400 million people across the globe deal with diabetes. An additional 200 million are expected to be diagnosed between now and 2040, and up to a third of these patients will likely develop diabetic retinopathy (DR)1.
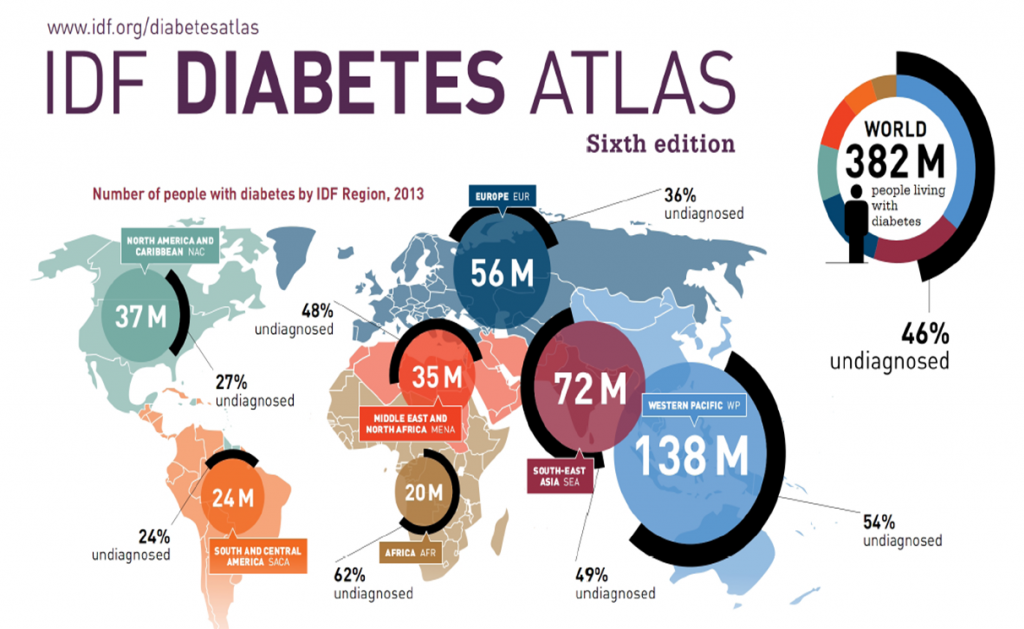
International Diabetes Federation (IDF) Atlas from 2013.
These increasing health challenges are driving the need for improved techniques to diagnosis and treat both DR and its common complications like diabetic macular edema (DME). One diagnostic tool – the visualization of the retinal periphery using ultra-widefield (UWF™) retinal imaging – is providing the ophthalmic community with important insights about the pathology, progression and treatment of DR and associated complications.
About UWF Retinal Imaging
UWF imaging is performed by a specially-designed scanning laser ophthalmoscope (SLO) that generates a high-resolution digital image capturing 200° or 82% of the retina. The SLO simultaneously uses two low-power lasers (red and green) that enable high-resolution, color imaging of retinal substructures.
Along with UWF color imaging, the technology also supports UWF fluorescein angiography (FA), UWF fundus autofluorescence (FAF), and UWF indocyanine green angiography (ICG).
Conventional 7 standard field (7SF) ETDRS photographs produce a comparatively narrow view (45° or less) of the center-portion of the retina. Peripheral portions are not imaged.
UWF Retinal Imaging’s Impact on DR and DME Treatment
The ability to quickly and reliably image the retinal periphery is providing new understanding about the progression of DR and the ability to design and test improved therapeutic regimens.
— Early diagnosis and treatment can limit DR progression. A recent study of diabetic patients2 over a four-year period used both UWF and conventional 7SF techniques to assess DR risk. Eyes where baseline 7SF grading provided no indication of DR, but which at the same time had lesions in the retinal periphery, were 2.5 times more likely to develop DR compared to those with no peripheral lesions (83% versus 33%). While confirmatory studies are needed, the results suggest that UWF imaging can enable new criteria for monitoring diabetic patients, and in so doing, diagnosis DR at earlier stages of disease progression.
— The use of UWF fluorescein angiography (UWF FA) to guide panretinal photocoagulation (PRP) is being tested3, creating the potential to minimize PRP application to retinal capillary nonperfusion and intermediate retinal ischemic affected zones, and in so doing, reduce the complications associated with PRP4.
DME treatment is also being impacted by UWF imaging. In one pilot study, DME patients with peripheral retina nonperfusion5 imaged via UWF FA were tested to assess the effectiveness of a single treatment with an intravitreal VEGF inhibitor (ranibizumab) plus UWF FA guided peripheral scatter laser treatment to the areas of nonperfusion. The underlying rationale for the study:
— Anti-VEGF (vascular endothelial growth factor) therapy, either alone or in conjunction with macular laser, is the standard of care for mitigating DME.
— The need for repeated anti-VEGF injections to maintain DME treatment benefits has suggested that persistent peripheral retinal ischemia may be driving VEGF production in a subset of patients.
— This subset – DME patients with the imaging signature of peripheral retinal nonperfusion – might benefit from UWF FA guided peripheral scatter laser therapy. The goal would be to diminish ischemia-driven VEGF production and with this reduce or eliminate the need for follow-on anti-VEGF therapy.
One of the study’s key findings was that after six months, 80% of the control group required rescue treatment as opposed to 33% of those in the experimental group. While not a definitive guide, this and other studies6 are developing new, targeted DME therapies made possible by UWF imaging techniques.

- Yau, Joanne W.Y, Rogers, Sophie L, et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care March 2012 vol. 35 no. 3 556-564
- Silva PS, Cavallerano JD, Sun JK, et al. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120(12):2587-2595.
- Muqit MM, Marcellino GR, Henson DB, et. al. Y Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol. 2013 May;91(3):251-8. doi: 10.1111/j.1755-3768.2011.02307.x. Epub 2011 Dec 16.
- Russ Rene, Levin Ariana, Kiss Szilard, Ultrawidefield Fluorescein Angiography in the Diagnosis of Diabetic Retinopathy, Retinal Physician, March 1, 2015; https://www.retinalphysician.com/articleviewer.aspx?articleID=112385#ref14
- Suner IJ, Peden MC, Hammer MK, et al. RaScaL: A pilot study to assess the efficacy, durability, and safety of a single intervention with ranibizumab plus peripheral laser for diabetic macular edema associated with peripheral non-perfusion on ultrawide-field fluorescein angiograpy [published online ahead of print November 26, 2014]. Ophthalmologica. doi:10.1159/000367902
- Tornambe P, London N. Is DME a peripheral retina disease which secondarily involves the macula? Paper presented at: Argentine Retina Society Meeting; November, 13-15, 2014; Mendoza, Argentina.