Retinal vein occlusion (RVO) is a restriction or blockage of blood flow leaving the retina and is the second most common retinal vascular disorder after diabetic retinopathy1. Causing varying degrees of vision loss, both central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO) can be complicated by macular edema that can lead to total blindness.
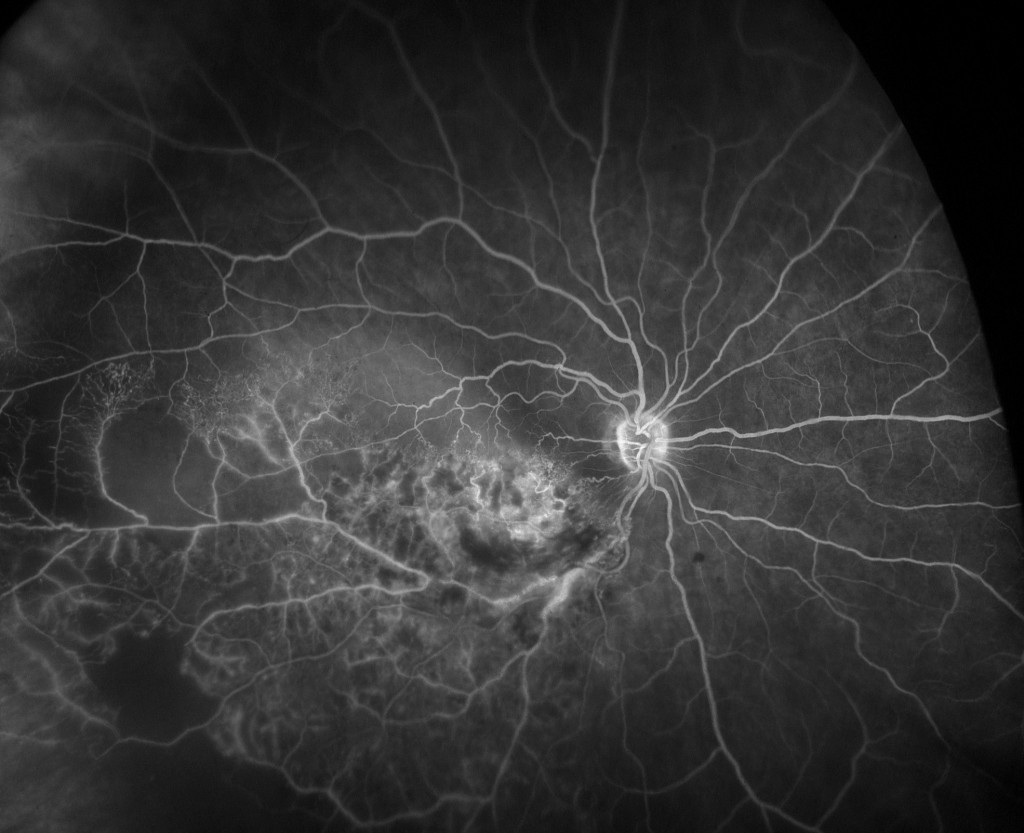
Branch retinal vein occlusion (BRVO) FA
RVO has a complex pathology, significant associated risk factors (e.g. age, hypertension, diabetic retinopathy, ischemic heart disease), and its treatment of associated macular edema may require no more than a single course treatment in some cases, but in others recurrent edema may necessitate one or more repeated courses of treatment.
One technology that is helping to advance the understanding and management of RVO is ultra-widefield retinal imaging (UWF™), which provides up to a 200 degree visualization of the retinal periphery. RVO is now being treated and studied using UWF retinal imaging, and data about the significance of RVO-associated pathology in the retinal periphery are being accumulated.
About Ultra-Widefield Retinal Imaging
UWF retinal imaging is performed by a specially designed scanning laser ophthalmoscope (SLO) that generates a high-resolution digital image covering 200° (or about 82%) of the retina. By comparison, conventional 7 standard field (7SF) ETDRS and fundus camera photographs produce a relatively narrow view (45° or less) of the central portion of the retina.
The SLO simultaneously images the retina using two low-power lasers (red and green) that enable high-resolution, color imaging of retinal substructures. The resulting UWF digital image – the optomap – is produced in a single capture, often without pupil dilation required. Along with UWF color imaging, the technology supports UWF fluorescein angiography (FA), UWF fundus autofluorescence (FAF), and UWF indocyanine green chorioangiography (ICG).
Characterization of Peripheral Retinal Nonperfusion in RVO Patients
A recent study2 utilized UWF to characterize peripheral retinal nonperfusion in RVO patients. The goal of the study was to understand the relationship of peripheral nonperfusion to the severity of macular edema and the response to treatment. A particular focus was trying to determine if peripheral ischemia could be the root cause of rebound edema after a course of intravitreal treatments for RVO. Large areas of peripheral nonperfusion may generate higher levels of vascular endothelial growth factor (VEGF) and in so doing increase the severity of macular edema. This could help answer questions about whether the extent of retinal ischemia (including peripheral ischemia), should influence RVO treatment decisions.
The study covered 32 patients, 13 of whom were diagnosed with central retinal vein occlusion (CRVO), and the remainder with branch retinal vein occlusion (BRVO). Study patients had previously received sequential treatment with intravitreal ranibizumab and dexamethasone implant followed within six months by recurrent, center-involved edema. Recurrence was defined by a foveal central subfield thickness > .300 um measured by OCT (optical coherence tomography).
Patients in the study were imaged using UWF fluorescein angiography (FA) at their enrollment, which coincided with a finding of recurrent macular edema. Follow-up retreatment(s) for the recurrences consisted of the same ranibizumab and dexamethasone combination referenced above, and patients were monitored monthly. A follow-up UWF FA image was made when the edema was deemed dry (OCT measurements < 300 um). An ischemic index, adapted for use with the UWF FA imaging, was used to estimate the percent of the total visible field exhibiting nonperfusion.
Among the study’s findings:
— For all patients in the study, areas of measured ischemia were lower when the edema was resolved – from an average of 14.8% at the start of the study to 10.3% with edema resolution.
— The above finding was also consistent among those with CRVO (22.5% versus 16.1%), and BRVO (11.0% versus 8.5%).
— There was a strong statistical relationship between the severity of the recurrent edema and measured ischemia. For those patients with an ischemia index below 10%, the mean central subfield thickness was 424.5um versus 520.8 um for those with an ischemic index above 10%.
—Response to treatment was remarkably similar among the groups with low and high levels of ischemia. Measured visual acuity improved to roughly similar levels, and foveal central subfield thickness results were actually somewhat better in the group with an ischemic index greater than 10% – a mean value of 224.5 um versus 257.4 um for the patients with less ischemia.
While the authors conceded that the small sample size of their study needs to considered in drawing conclusions about their results, they observed that:
— The positive treatment results for patients with a high degree of nonperfusion were of note, as many previous clinical trials for RVO treatments excluded patients with significant nonperfusion. These same clinical trials did not use UWF imaging, suggesting that significant nonperfusion may have been present, but undetected.
— Individuals with severe edema measured by OCT may well be candidates for UWF follow-up to identify any nonperfusion and possible neovascular complications.
The authors speculated that the improvement in nonperfusion after treatment indicates that some portion of the circulation in the affected area is not permanently impaired, is modulated by VEGF, and so responds to anti-VEGF medication.
In an associated article, the authors suggested that in cases where patients suffer one or more instances of rebound edema over the course of treatment, the use of targeted panretinal photocoagulation (PRP) on portions of the retina where circulation is permanently impaired could decrease the patients’ VEGF load and break the cycle of rebound edema. They have begun reporting on some of the early results of this RVO treatment strategy.

- Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina.2013; 33(5):901–91. PMID:23609064.
- Singer M, Tan CS, Bell D, et al. Area of peripheral retinal nonperfusion and treatment response in branch and central retinal vein occlusion. Retina. 2014;34(9):1736-1742.